When scientists announced promising interim results from the first large-scale clinical trial of a possible vaccine for malaria, the international public health community rejoiced. The vaccine, originally a project for the U.S. military, is known as RTS,S. It is a collaboration between GlaxoSmithKline (GKL), PATH Malaria Vaccine Initiative (MVI), and the Bill & Melinda Gates Foundation.
Over 15,000 African children in 7 African countries are participating in the trial. Among 6,000 children ages 5-17 months old, it reported a 47% reduction in malarial cases within a year. While this marks a great public health achievement, other vaccine researchers have identified several concerns on the results that, they point out, are still preliminary.
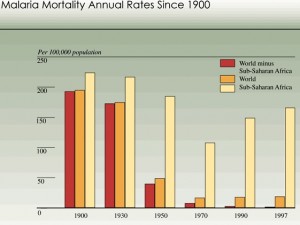
The World Health Organization (WHO) estimates that malaria infects 191-311 million people and kills 708,000-1,003,000 of them, the majority of which are African children. Children suffer the burden of the disease because their immune systems are still developing.
The vaccine has been in development for over 30 years because of the disease’s complexities: the malaria species are multi-stage parasites capable of evolutionary change, which makes it difficult for scientists to develop an effective vaccine. These multiple stages mean that a vaccine would only induce immune responses specific to each stage, so any one parasite that eludes the response would be enough to avoid the vaccine-induced protection.
Also, complete immunity from malaria is rare. Vaccines for many bacterial and viral infections only need one natural infection to induce long-lasting immunity. But even after several malaria infections, a vaccine is unable to mimic immunity that should be obtained from natural infection. These difficulties have not stopped malaria experts from trying to find treatments for malaria cases around the world.
The Center for Disease Control (CDC) does list preventative measures such as insecticide-treated nets, vector control, and indoor residual spraying proven to be highly effective in many parts of the world. Unfortunately, malaria-ridden Sub-Saharan Africa would not respond to precautionary tools because of the high transmission rates. Additionally, there has been massive resistance efforts to the practical use of DDT and other insecticides to control malaria, prompting alternative non-insecticide-based mosquito control measures. These led to the need for the development of a vaccine that would work in conjunction with existing treatments to eradicate malaria.

The production of effective malaria vaccines has lacked progress in the past, but the new pilot vaccine reveals positive results. RTS,S would be given to children at the same time as other routine immunizations. This would make distribution more accessible.
The vaccine acts more by reducing the rate of new infections, rather than combating current infection. And as mentioned, it was somewhat effective in the trials: the total number of clinical episodes of malaria was reduced to 55%. But despite this, the clinical trial has undergone much scrutiny.
By the end of 2014, the trial will be completed and all results may be assessed. But the decision to publish partial data attracted criticism from leading vaccine researchers. Interim data is usually only reported to regulatory authorities, and the premature publishing brings into question the results’ validity especially with “less than half the efficacy results available,” writes Nicholas White, a malaria researcher in Bangkok.
Also, Stephen Hoffman, chief executive of a rival vaccine effort based in Maryland, mentions that the publication fails to report data on children aged 6-12 weeks, which was the stated target group of the trial. The 47% reduction in severe malaria, combined with available data on the younger age group, brings the new efficacy down to 35%. This low efficacy rate would represent a negligible benefit to the younger age group and fall short of the 50% protective efficacy rate mandated by the WHO in 2006 to win approval.
A third issue lies in the vaccine’s long-term protection. The 47% reduction was reported in just a year’s time span. Researchers will not be able to identify the length of time in which the vaccine protects against malaria until all data has been compiled.
In a live chat with two leading malaria experts–Laurence Slutsker and Vasee Moorthy from WHO– the question of the vaccine’s partial protection was brought up. They said that the vaccine should work in tandem with current control measures to bring morbidity and mortality rates to zero.
In response to a question about potential side-effects, they responded that, “to date, the vaccine has had an acceptable safety and tolerability profile.” A higher rate of meningitis in vaccinated children compared to controls did not arouse serious concern because it was statistically insignificant. The length of time between vaccination and disease onset also suggested no relation between the two events. However, the vaccine will continue to be monitored for adverse effects over the rest of the trial.
Regardless of the trial’s criticisms, the global implications of the first-generation vaccine are remarkable and provides a stepping-stone not only for malaria control, but global health as well. RTS,S marks the first vaccine to consistently provide a significant protective effect against a parasite on a large-scale setting.GSK promises to provide the vaccine at an affordable price, just 5% above the cost of production. Excess profit would be reinvested into developing the next malaria vaccine.
While researchers did hope for a higher malarial reduction rate than 50%, the vaccine alone has the potential to prevent millions of malaria cases in children. With the powerful combination of the vaccine and current malaria control measures, there is hope. Controlling malaria would help many developing nations’ economies for which the disease accounts for 40% of medical costs.
The RTS,S vaccine is not perfect, but it shows much promise as a first step. If given regulatory approval, this would be the first human vaccine against a parasitic disease. With full results expected in 2014, public health authorities patiently await the announcement of what could be the first effective vaccine against malaria, the second leading cause of death from infectious diseases in Africa.